Disease Overview
hATTR amyloidosis is a multisystem, rapidly progressive, often fatal disease1-3
Hereditary transthyretin-mediated (hATTR)
amyloidosis is an autosomal dominant disease
caused by one of many possible variants in the transthyretin (TTR) gene.3 An estimated 50,000 people are living with hATTR amyloidosis worldwide.4,5
How hATTR amyloidosis develops
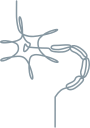
Formation of amyloid deposits1,2,6


TTR TETRAMERS
TTR is primarily synthesized in the liver and is secreted as a tetramer.


TTR MONOMERS
In hATTR amyloidosis, a genetic variant causes the tetramer to become less stable, resulting in dissociation into monomers.


MISFOLDED TTR
TTR monomers misfold and aggregate into amyloid deposits.


AMYLOID DEPOSITS
Amyloid is deposited
at multiple sites in the body, causing damage that leads to clinical symptoms.

PROGRESSIVE SYMPTOMS
Accumulation of amyloid deposits over time results in worsening clinical symptoms.
Another type of ATTR amyloidosis is called wild-type ATTR amyloidosis. The etiology is unknown, but is presumed to be associated with aging.1,7
The multisystem nature of hATTR amyloidosis takes a toll on the whole body1
Multisystem involvement and family history are red flags of hATTR amyloidosis and require urgent action.3 If your patient’s family has a history of the disease
or of its many symptoms, or your patient is experiencing a combination of multisystem symptoms, the cause could be hATTR amyloidosis.


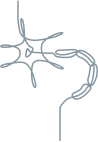
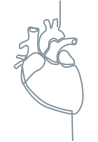

Sensory-motor neuropathy1,3
-
Length-dependent neuropathic pain and numbness
-
Altered sensation
-
Weakness
-
Difficulty walking
-
Bilateral carpal tunnel syndrome
Autonomic neuropathy1,3
-
Orthostatic hypotension
-
Diarrhea, constipation, nausea and vomiting
-
Unintentional weight loss
-
Recurrent urinary tract infections
-
Sexual dysfunction
Cardiac manifestations3,8
-
Conduction abnormalities
-
Arrhythmias
-
Heart failure
-
Left ventricular hypertrophy
Additional findings3,8
-
Family history of hATTR amyloidosis symptoms or diagnosis
-
Rapid symptom progression
-
Failure to respond to immunomodulatory treatment
-
Intolerance of commonly used cardiovascular medications
Progression of polyneuropathy leads to significant disability22



Progression of sensory-motor neuropathy1,10-121,9-11
-
Sensory loss can reduce dexterity and temperature sensation
-
Motor deficits result in progressive weakness and impaired ambulation
-
Sensory-motor neuropathy can progress more than 10x faster than diabetic neuropathy
Progression of autonomic neuropathy11-1910-19
-
Orthostatic hypotension can cause syncope and sudden falls
-
Gastrointestinal issues can cause patients to isolate themselves from social situations
-
Continuous weight loss
leads to wasting and
reduced survival -
Autonomic neuropathy can induce fatal arrhythmias
Progression of cardiac manifestations8,20-228,20-22
-
Significant and measurable decline in cardiac function results in heart failure
-
Heart failure due to hATTR amyloidosis progresses
more quickly than with other cardiac conditions
Not a comprehensive list of all the symptoms associated with hATTR amyloidosis.
Each patient may not experience all of these symptoms or may not experience them at the same time.
Multisystem dysfunction is a reality for most patients1
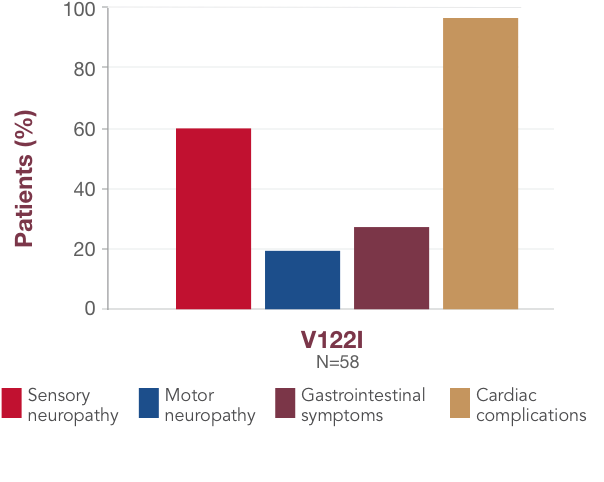
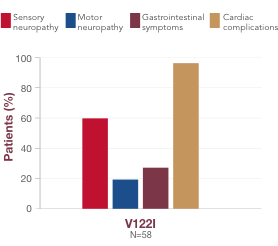
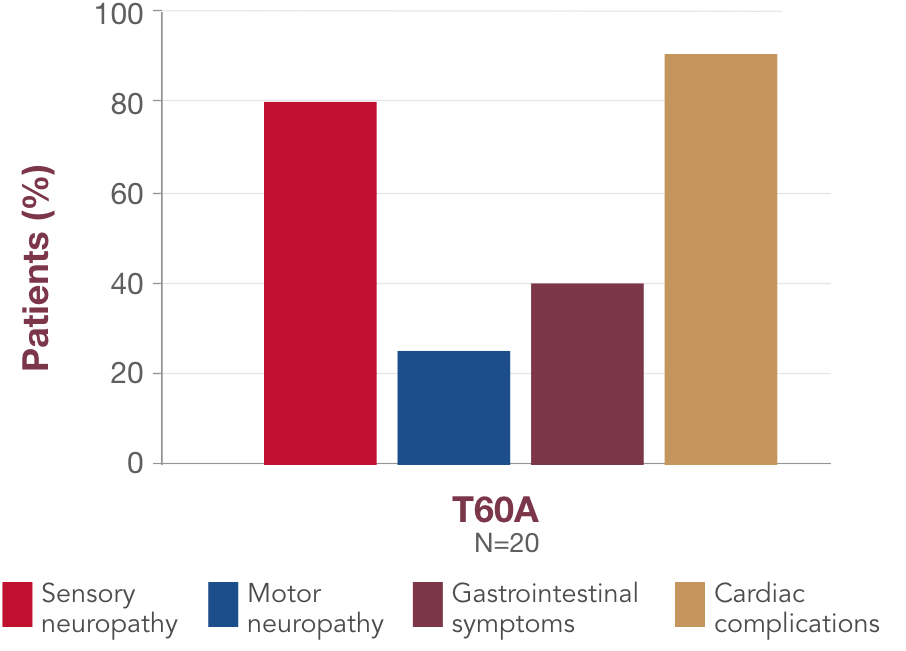
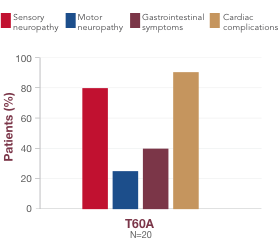
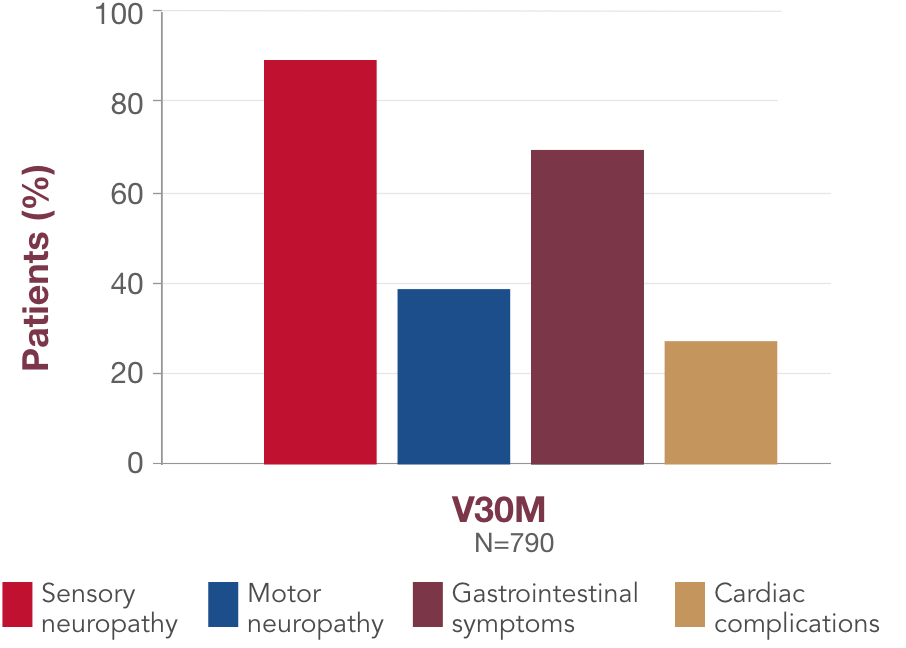
Although there is some association between variant and symptom presentation, most patients suffer from overlapping symptoms of sensory-motor neuropathy, autonomic neuropathy, and cardiac manifestations.1 In the United States, the most common variants (V122I, T60A, and V30M) are associated with both polyneuropathy and cardiomyopathy.1,19,23,241,18,23,24 Polyneuropathy may precede or coincide with cardiomyopathy, even in patients with the V122I variant.25
bData collected by the THAOS registry.
THAOS=Transthyretin-Associated Amyloidosis Outcomes Survey.
Patients with hATTR amyloidosis may already be in your practice
In the profiles below, you will find examples of
different patient types, which may help you to
recognize patients in your practice who may
be at
risk of hATTR amyloidosis. Patient profiles are composites created through a review of published literature and are not of actual patients.

Patient Resources
Family Resources
Information and Support Groups
References:
- Ando Y, Coelho T, Berk JL, et al. Orphanet J Rare Dis. 2013;8:31.
- Adams D, Coelho T, Obici L, et al. Neurology. 2015;85(8):675-682.
- Conceição I, González-Duarte A, Obici L, et al. J Peripher Nerv
Syst. 2016;21(1):5-9. - Plante-Bordeneuve V. J Neurol. 2014;261(6):1227-1233.
- Hawkins PN, Ando Y, Dispenzeri A, et al. Ann Med.
2015;47(8):625-638. - Kourelis TV, Gertz MA. Expert Rev Cardiovasc Ther.
2015;13(8):945-961. - Gertz MA. Am J Manag Care. 2017;23(suppl 7):S107-S112.
- Dharmarajan K, Maurer MS. J Am Geriatr Soc. 2012;60(4):765-774.
- Berk JL, Suhr OB, Obici L, et al. JAMA. 2013;310(24):2658-2667.
- Shin SC, Robinson-Papp J. Mt Sinai J Med. 2012;79(6):733-748.
- Koike H, Tanaka F, Hashimoto R, et al. J Neurol Neurosurg
Psychiatry. 2012;83(2):152-158. - Wixner J, Mundayat R, Karayal ON, et al. Orphanet J Rare Dis. 2014;9:61.
- González-Duarte A, Berk JL, Quan D, et al. J Neurol. 2020;267(3):703-712.
- González-Duarte A. Clin Auton Res. 2019;29(2):245-251.
- Ando Y, Suhr OB. Amyloid. 1998;5(4):288-300.
- Suhr O, Danielsson A, Holmgren G, et al. J Intern Med. 1994;235(5):479-485.
- Low PA. Clin Auton Res. 2008:18(suppl 1):8-13.
- Castaño A, Drachman BM, Judge D, et al. Heart Fail Rev. 2015;20(2):163-178.
- Ruberg FL, Maurer MS, Judge DP, et al. Am Heart J.
2012;164:222-228. - Olivotto I, Cecchi F, Pogessi C, et al. Circ Heart Fail.
2012;5(4):535-546. - Drazner MH. Circulation. 2011;123(3):327-334.
- Coutinho P, Martins da Silva A, Lopes Lima JL, et al. Excerpta
Medica. 1980:88-98. - Maurer MS, Hanna M, Grogan M, et al. J Am Coll Cardiol. 2016;68(2):161-172.
- Parman Y, Adams D, Obici L, et al. Curr Opin Neurol. 2016;29(suppl 1):S3-S13.
- Grogan M, Hawkins PN, Kristen AV, et al. Poster presented at: 23rd Annual Meeting of the Heart Failure Society of America (HFSA); September 13-16, 2019; Philadelphia, PA.
- Jacobson D, Tagoe C, Schwartzbard A, et al. Am J Cardiol. 2011;108(3):440-444.
- Parker MM, Damrauer SM, Rader DJ, et al. Presented at: AANEM Annual Meeting; October 10-13, 2018.
- Reilly MM, Staunton H, Harding AE, et al. J Neurol Neurosurg
Psych. 1995;59(1):45-49. - Sattianayagam PT, Hahn AF, Whelan CJ, et al. Eur Heart J. 2012;33:1120-1127.
- Adams D, Ando Y, Beirao JM, et al. J Neurol. 2021;268(6):2109-2122.
- Gillmore JD, Maurer MS, Falk RH, et al. Circulation.
2016;133(24):2404-2412. - Maurer MS, Elliott P, Comenzo R, et al. Circulation.
2017;135(14):1357-1377. - Adams D, Suhr OB, Hund E, et al. Curr Opin Neurol. 2016;29(suppl 1):S14-S26.